Background: Venetoclax (VEN) in combination with hypomethylating agents (HMA) or low dose cytarabine (LDAC) is the standard of care for the treatment of newly diagnosed AML in pts aged ≥ 75 yrs. or unfit for intensive chemotherapy. While considered low-intensity, myelosuppression is universal, leading to increased rates of infection in the setting of neutropenia. Antifungal prophylaxis during induction has significantly lowered treatment-related mortality (TRM) attributed to invasive fungal infections (Cornely et al, 2007). Azole antifungals, the treatment of choice, pose significant drug-drug interactions through inhibition of CYP3A4 and p-glycoprotein (P-gp), both responsible for VEN metabolism and clearance. This interaction alters VEN pharmacokinetics (PK), increases its exposure, and therefore requires substantial dose adjustments (Agarwal et al, 2017). We previously showed that dose adjusted VEN plus HMA when used concomitantly with azoles retains efficacy, but still resulted in prolonged myelosuppression (Rausch et al, 2022). Little real-world PK data exists to confirm whether the recommended dose adjustments are appropriate or require further refinement.
Methods: Pharmacokinetic analysis was conducted during the induction cycle of the phase II study of cladribine plus LDAC and VEN as previously described (Kadia et al, 2022). VEN was administered on days 1-21 during induction and was dose-adjusted to either 100 mg (later amended to 50 mg), 100 mg, or 400 mg daily when used with posaconazole (posa), voriconazole (vori), or caspofungin (caspo), respectively. Dose adjustments were based on package insert recommendations. Prior to amendment, VEN 100 mg was administered with posa based on previously published PK analysis suggesting a 75% dose reduction of VEN with concomitant posa (Agarwal et al, 2017). Steady state VEN PK analysis was conducted on day 8 and trough levels were collected on days 12 and 16 of cycle 1. Levels obtained with concomitant caspo served as a reference level, assuming no significant interaction between VEN and caspo. VEN levels were analyzed via an institutionally developed liquid chromatography coupled mass spectrometry (LCMS) assay and PK analysis was conducted via non-compartmental method using WinOnlin.
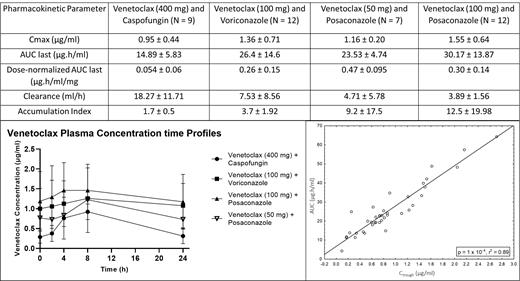
Results: Forty patients, median age 68 (range, 61-78), were included for pharmacokinetic analysis. Average VEN AUC (area under the exposure) and Cmax (maximum observed plasma concentration) was higher with concomitant vori or posa than with caspo regardless of dose adjustments. Posa in combination with VEN 100 mg increased average AUC by 103% and Cmax by 63%. Vori with VEN 100 mg increased AUC and Cmax by 77% and 43%, respectively. Among the 3 groups, posa with VEN 50 mg resulted in a VEN AUC and Cmax most similar to that observed with caspo and VEN 400 mg. Concomitant posa delayed VEN clearance by 74% with VEN 50 mg and 79% with VEN 100 mg. As a result, the accumulation index observed with posa was 12.5 + 19.98 with VEN 100 mg, 9.2 + 17.5 with VEN 50 mg, and 3.7 + 1.92 with vori, compared to 1.7 + 0.5 with caspo. Although there was interpatient variability in VEN trough levels, we observed consistent intra-patient trough levels. In addition, significant correlation between VEN AUC and VEN trough levels (p = 1 x 10 -4, r 2 = 0.89) was observed.
Conclusions: Despite dose adjustments, VEN AUC is significantly higher among pts receiving azole antifungals compared to those receiving caspo. Posa resulted in more potent inhibition of VEN clearance and greater accumulation compared to vori, despite both agents considered strong CYP3A4 inhibitors. This may be a result of the dual inhibitory effect of posa on both CYP3A4 and P-gp activity or more potent inhibition of CYP3A4. Based on PK evaluation, our data suggest VEN 50 mg with concomitant posa provides more similar drug exposure to VEN 400 mg and may be more appropriate than 70 mg or 100 mg in this setting. Given the high accumulation index observed with posa, PK analyses during cycle 2 are ongoing. While there is inter-patient variability in VEN PK, intra-patient trough levels are consistent, correlate well with VEN AUC, and could be used for therapeutic drug monitoring.
Disclosures
DiNardo:Fogham: Honoraria; Servier: Honoraria; Schrödinger: Consultancy; Notable Labs: Honoraria; Novartis: Honoraria; Takeda: Honoraria; AbbVie/Genentech: Honoraria; ImmuniOnc: Honoraria; Astellas: Honoraria; BMS: Honoraria. Ravandi:Prelude: Research Funding; Amgen: Honoraria, Research Funding; Xencor: Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Syros: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Biomea fusion: Honoraria, Research Funding; Astex/taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding. Daver:Pfizer: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Agios: Consultancy; Syndax: Consultancy; Gilead: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; AROG: Consultancy; Trovagene: Research Funding; ImmunoGen: Consultancy, Research Funding; Servier: Consultancy, Research Funding; Shattuck Labs: Consultancy; FATE: Research Funding; Jazz: Consultancy; Glycomimetics: Research Funding; Novartis: Consultancy; Novimmune: Research Funding; Amgen: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Hanmi: Research Funding; Kite, a Gilead company: Consultancy, Research Funding; Celgene: Consultancy; Astellas: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Kronos Bio: Research Funding. Borthakur:Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Pacylex, Novartis, Cytomx, Bio Ascend:: Membership on an entity's Board of Directors or advisory committees; Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy. Pemmaraju:Physician Education Resource (PER): Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Imedex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dava Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; OncLive: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ImmunoGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Intellisphere: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Harborside Press: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Menarini Group: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Medscape: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Magdalen Medical Publishing: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CancerNet: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aplastic Anemia & MDS International Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CareDx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASH Committee on Communications: Other: Leadership; PeerView Institute for Medical Education: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Pacylex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Neopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Curio Science: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Patient Power: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ClearView Healthcare Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Protagonist Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; ASCO Cancer.Net Editorial Board: Other: Leadership; Karger Publishers: Other: Licenses; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cimeio Therapeutics AG: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; United States Department of Defense (DOD): Research Funding; National Institute of Health/National Cancer Institute (NIH/NCI): Research Funding; HemOnc Times/Oncology Times: Other: Uncompensated; Bristol Myers Squibb Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aptitude Health: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Short:AstraZeneca: Consultancy; Astellas: Research Funding; Novartis: Consultancy; Takeda: Consultancy, Research Funding; Stemline therapeutics: Research Funding; Amgen: Honoraria; Pfizer: Consultancy. Jabbour:Pfizer: Consultancy, Honoraria, Research Funding; Ascentage Pharma Group: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Adaptive Biotech: Consultancy, Honoraria, Research Funding; Hikma Pharmaceuticals: Consultancy, Honoraria, Research Funding. Garcia-Manero:Bristol Myers Squibb: Other: Medical writing support, Research Funding; Genentech: Research Funding; AbbVie: Research Funding. Kantarjian:Abbvie (Inst): Research Funding; Taiho Pharmaceutical: Honoraria; Shenzhen Target Rx: Honoraria; Precision Biosciences: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; KAHR Medical: Honoraria; Jazz Pharmaceuticals (Inst): Honoraria, Research Funding; Ipsen: Honoraria; Immunogen (Inst): Honoraria, Research Funding; Daiichih-Sankyo (Inst): Honoraria, Research Funding; AstraZeneca/MedImmune: Honoraria; Astellas Pharma: Honoraria; Ascentage Pharma Group: Honoraria; Amgen: Honoraria; Abbvie: Consultancy, Honoraria; Novartis (Inst): Research Funding; Bristol-Myers Squibb (Inst): Research Funding; Amgen (Inst): Research Funding; Ascentage Pharma (Inst): Research Funding. Kadia:AstraZeneca: Research Funding; Sanofi-Aventis: Consultancy; Genzyme: Honoraria; Celgene: Research Funding; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; Novartis: Consultancy; Iterion: Research Funding; Liberum: Consultancy; GenFleet Therapeutics: Research Funding; Cellenkos Inc.: Research Funding; Cyclacel: Research Funding; Astellas Pharma Global Development: Research Funding; Cure: Speakers Bureau; Glycomimetics: Research Funding; Hikma Pharmaceuticals: Speakers Bureau; Genentech: Consultancy, Research Funding; Regeneron Pharmaceuticals: Research Funding; Delta-Fly Pharma, Inc.: Research Funding; SELLAS Life Sciences Group: Research Funding; Pulmotect, Inc.: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Janssen Research and Development: Research Funding; Ascentage Pharma Group: Research Funding; Amgen, Inc.: Research Funding; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; Agios: Consultancy; Servier: Consultancy; Pinotb-Bio: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal